what atrial wall muscle fails to contract in a synchronous fashion during atrial fibrillation?
| A) | bundle of His. | ||
| B) | sinoatrial node. | ||
| C) | Purkinje arrangement. | ||
| D) | atrioventricular node. |
A BRIEF REVIEW OF NORMAL ELECTRICAL CONDUCTION
In the normal heart, the heart beat is initiated past the sinoatrial (SA) node. From the SA node, the electrical impulse travels through both the right and left atria, causing depolarization of the atria. Atrial depolarization is followed by atrial wrinkle and atrial repolarization. The electrical impulse travels from the atria to the atrioventricular (AV) node located in the inferior wall of the correct atrium. The speed of conduction slows in the AV node to allow time for the atria to depolarize, contract, and consummate ventricular filling. From the AV node, the electric impulse travels through the bundle of His located in the septum of the heart. The bundle of His divides into the right and left bundle branches. These branches dissever further into the smaller fibers of the Purkinje system. Electric conduction through the His-Purkinje organisation is rapid, causing rapid depolarization of both the right and left ventricles. Depolarization of the ventricular cells spreads from the noon of each ventricle to the base, and moves from the endocardium to the epicardium. Ventricular depolarization is followed by ventricular contraction and ventricular repolarization [10,11,12].
2 . A prolonged QT interval indicates
| A) | loss of atrial wrinkle. | ||
| B) | prolonged ventricular repolarization. | ||
| C) | shortened atrial and ventricular refractory periods. | ||
| D) | accelerated, abnormally rapid depolarization of the ventricles. |
A REVIEW OF ELECTROCARDIOGRAM WAVEFORM
When changes occur in the normal cardiac cycle, the normal ECG waveform is altered to reflect them. For example, prolonged repolarization is reflected in a prolonged QT interval. A slowing of conduction from the SA node through the AV node may be reflected in a prolonged PR interval. Abnormal conduction of the electrical impulse through the ventricles results in a QRS interval that is wider than usual or bizarre in shape. Careful analysis of the changes in a patient's ECG tin can provide valuable data in the diagnosis and treatment of the arrhythmia [seven,16].
3 . Of the following, which is a key defining characteristic of atrial fibrillation?
| A) | A prolonged QRS interval | ||
| B) | A ventricular rate in excess of 300 bpm | ||
| C) | Presence of clearly divers, regular sinus P waves | ||
| D) | An irregularly irregular ventricular response when conduction through the AV node is normal |
ATRIAL FIBRILLATION
The ii central defining characteristics of atrial fibrillation are ( Effigy two ) [three,6,17,xix]:
-
Total absence of normal sinus P waves. The absence of P waves indicates that the middle beat was not initiated in the SA node, or normal pacemaker, of the heart. The P waves are replaced by fibrillatory (fib) waves. These fib waves may be so fine that they are indiscernible or barely discernible; or, they may exist very coarse and more clearly seen on the ECG tracing.
-
An irregularly irregular ventricular response when conduction through the AV node is normal.
4 . A central feature that tin can differentiate AV nodal re-aspirant tachycardia from atrial fibrillation is
| A) | the rhythm in AV nodal re-entrant tachycardia is regular. | ||
| B) | sinus P waves are absent-minded in AV nodal re-entrant tachycardia. | ||
| C) | the charge per unit in AV nodal re-entrant tachycardia rarely exceeds 120 bpm. | ||
| D) | the QT interval in AV nodal re-entrant tachycardia is always lengthened. |
ATRIAL FIBRILLATION
In AV nodal re-aspirant tachycardia (also referred to equally paroxysmal supraventricular tachycardia or PSVT), the ventricular rate falls between 150 and 250 bpm. No evidence of atrial activity is present. The ventricular rhythm is regular. The arrhythmia has an sharp onset and termination, with episodes lasting from seconds or minutes to days, and may occur in persons with no history of heart disease as well as in elderly persons with chronic heart affliction. The arrhythmia may be differentiated from sinus tachycardia past rate; sinus tachycardia rarely exceeds a rate of 150 to 160 bpm in an developed at rest. It may also be differentiated from atrial fibrillation because the rhythm is regular. Information technology may exist differentiated from atrial palpitate past rate; the usual rates associated with AV nodal re-entrant tachycardia are too slow for 1:1 (atrial to ventricular) conduction in atrial flutter and as well fast for ii:one conduction [three,17,25].
5 . Cardiac problems linked to the development of atrial fibrillation include which of the post-obit?
| A) | Hypertension | ||
| B) | Mitral valve disease | ||
| C) | Rheumatic center disease | ||
| D) | All of the above |
ATRIAL FIBRILLATION
Every bit a secondary arrhythmia, atrial fibrillation may be caused by cardiac and noncardiac causes. Common cardiac causes include [30,31,32]:
-
Hypertension
-
Rheumatic centre disease
-
Mitral valve disease (e.g., mitral stenosis, mitral valve prolapse, mitral valve annular calcification)
-
Congestive cardiomyopathy/congestive eye failure
-
Acute myocardial infarction
-
Sick sinus syndrome
-
Pericarditis
-
Hypertrophic cardiomyopathy
-
May occur following cardiac/coronary artery bypass graft (CABG) surgery
vi . One noncardiac cause linked to the development of atrial fibrillation is
| A) | diabetes. | ||
| B) | psoriasis. | ||
| C) | rapid weight gain or loss. | ||
| D) | normal renal and hepatic function. |
ATRIAL FIBRILLATION
The persons at highest risk to develop atrial fibrillation are those with long-standing hypertension, valvular heart disease, left ventricular hypertrophy, depressed left ventricular function, and coronary avenue illness. Atrial fibrillation associated with cardiovascular affliction may initially have a paroxysmal onset; however, the arrhythmia tin keep to progress to persistent or chronic atrial fibrillation. Noncardiac, systemic diseases may also cause atrial fibrillation. Diabetes mellitus is a major hazard gene for the development of atrial fibrillation. Other noncardiac causes include [2,30,31,32]:
-
Hyperthyroidism
-
Male sex
-
Advancing age
-
Obesity
-
Obstructive sleep apnea
-
Genetic factors
-
Alcohol and drug use
-
Noncardiac surgery
-
Noncardiac diagnostic procedure
-
Pulmonary conditions/hypoxemia acquired by pulmonary weather (e.grand., pneumonia, chronic obstructive pulmonary disease [COPD])
-
Pulmonary embolus
-
Over-the-counter use of some herbs, such equally ephedra or ginseng
vii . A physical assessment finding commonly associated with atrial fibrillation is
| A) | slowed heart charge per unit. | ||
| B) | diastolic hypertension. | ||
| C) | variable pulse pressure level. | ||
| D) | regular jugular venous pulsations. |
ASSESSMENT OF THE PATIENT WITH ATRIAL FIBRILLATION
Physical assessment findings in atrial fibrillation may include the following [iii]:
-
Rapid centre charge per unit and irregularly irregular center rhythm
-
Irregular jugular venous pulsations
-
Variable loudness of S1
-
Variable pulse pressure. This results from the variable ventricular filling acquired by the irregular conduction of atrial impulses through the AV node to the ventricles.
-
A claret pressure that appears to vary widely. In atrial fibrillation with a controlled or slow ventricular response, in that location may be long pauses betwixt some beats. When an electronic, noninvasive blood pressure device is used (or when the pressure is released too rapidly during transmission auscultation of blood pressure), the systolic blood pressure reading may vary widely. Taking serial blood pressure readings and using an boilerplate of readings to estimate the patient'south actual blood pressure may be needed.
-
Hypotension, especially if cardiac output is significantly reduced
-
Signs of congestive center failure, such as decreased oxygen saturation and rales/crackles in lung fields
-
Signs of poor peripheral perfusion, such as macerated peripheral pulses and dumb capillary filling
8 . The initial goal for a patient with atrial fibrillation with a rapid ventricular response is
| A) | rate command. | ||
| B) | reduction of elevated blood pressure. | ||
| C) | improvement of pulmonary hypertension. | ||
| D) | increased conduction beyond the AV node. |
ESTABLISHING THE MEDICAL Programme OF CARE
Based on a thorough evaluation of the patient'southward status and other factors, one or more medical goals should be identified. The initial goal for a patient who is hemodynamically unstable is the immediate restoration of normal sinus rhythm through electrical cardioversion. The initial goal for patients who nowadays with atrial fibrillation with a rapid ventricular response is rate control. Once the patient'due south status has stabilized, long-term goals may be developed. When developing long-term goals, consider the following points [three,17,23,33,38,39,40,41,42]:
-
Utilise of antiarrhythmic therapy may not be necessary for persons with asymptomatic paroxysmal atrial fibrillation.
-
Use of antiarrhythmic therapy is indicated for persons who experience severe symptoms with paroxysmal atrial fibrillation.
-
Long-term rate control is indicated for persons with persistent or permanent atrial fibrillation.
-
Long-term charge per unit control is also indicated for patients who have repeatedly reverted to atrial fibrillation following electrical or pharmacologic cardioversion. Ablation for symptomatic persistent atrial fibrillation and for severely symptomatic recurrent atrial fibrillation may be indicated.
-
Catheter ablation performed in experienced centers may be indicated to maintain sinus rhythm in select patients with significantly symptomatic, paroxysmal atrial fibrillation who have failed treatment with an antiarrhythmic drug and accept normal or mildly dilated left atria, normal or mildly reduced LV function, and no severe pulmonary disease.
-
Catheter ablation is indicated for symptomatic patients with atrial fibrillation who have Wolff-Parkinson-White (WPW) syndrome.
-
Restoration of normal sinus rhythm is indicated for those who take persistent signs of decreased cardiac output during episodes of atrial fibrillation.
-
Directly-current cardioversion may be indicated as part of a long-term management strategy to restore sinus rhythm in patients with atrial fibrillation.
-
Maintenance of normal sinus rhythm may be indicated for persons who spontaneously catechumen from atrial fibrillation to sinus rhythm.
-
Maintenance of normal sinus rhythm is indicated for persons who are successfully converted by pharmacologic or electrical ways.
-
Elimination or interruption of the arrhythmia through radiofrequency ablation (of the focal source of the arrhythmia or the AV node) is indicated for patients who cannot tolerate antiarrhythmic therapy, whose arrhythmia is not successfully controlled by optimal doses of antiarrhythmic therapy, or who cannot be successfully cardioverted through pharmacologic or electrical means.
9 . To receive FDA approval, a generic medication must run across all of the post-obit criteria, EXCEPT:
| A) | The generic drug must incorporate the same amount of agile drug ingredient equally the proprietary version. | ||
| B) | The generic drug must be released at the same rate and to the same extent as the proprietary version. | ||
| C) | The generic drug must be manufactured according to federal standards defined in the Good Manufacturing Practices. | ||
| D) | The generic and proprietary drugs must accept identical inactive ingredients, including fillers, binders, and preservatives. |
ESTABLISHING THE MEDICAL Programme OF Care
When a pharmaceutical company develops a new drug, the company may utilize for one or more than patents for (1) the drug itself; (2) the manufacturing process; (iii) how the drug is delivered to the bloodstream; or (4) how the medication is to be used. Although the patent gives the company exclusive rights to the new drug for 17 years, this flow ofttimes involves at to the lowest degree ten years of development. In reality, the company may take only seven years to exclusively sell the drug. A newly developed drug is given several names. The generic name, which is the medication's official name (derived from the drug's chemical name, structure, and/or formula), must be unique. Generic names are oftentimes difficult to pronounce, remember, and spell. The new drug is also given a trade name or proprietary name that signifies that the drug is the exclusive property of its visitor. Trade names are simpler, easier to recall, and frequently emphasize an attribute of the medication. Merchandise names must as well be unique. Subsequently the patent on a specific drug has expired, other companies may manufacture and sell that drug under its generic name. Generics are frequently sold at a lower price than merchandise/proprietary drugs. Generic versions of a drug must run across FDA approval, specifically the following three points [44,45]:
-
The generic training must contain the same amount of active drug ingredient as the original proprietary preparation.
-
The generic must be manufactured according to federal standards equally divers in the Good Manufacturing Practices.
-
In the homo trunk, the generic medication must exist released in equivalent fashion (i.e., same rate, to aforementioned extent) equally the proprietary drug. This is referred to as "bioequivalence." Bioequivalence is established by a drug company through the employ of minor research studies. For time-release medications, the process of establishing bioequivalence is more strict, extensive, and fourth dimension-consuming. Because there is more than variation inherent in the use of fourth dimension-release forms, more extensive testing is required to ensure bioequivalence. Considering of the cost and extensiveness of the procedure, very few time-release generic drugs are available.
Proprietary and generic versions of a medication may vary in several respects [26,45]:
-
Advent of the medication. By law the size, colour, and shape of the generic must significantly differ from the proprietary. Patients will discover the difference.
-
Different inactive ingredients. While the active ingredients must be the same, the inactive components may vary. Inactive ingredients are routinely used in medications to add bulk, to keep the tablet from crumbling/disintegrating until apply, to help the medication deliquesce, or to provide a pleasant taste. In that location have been instances in which the departure in inactive ingredients has changed the absorption of the agile ingredients.
-
Variable bioequivalence. Regulations permit equally much as a 20% variation in bioequivalence. For medications, such as antiarrhythmic medications that may accept a very narrow margin for therapeutic effect, this variation may alter how effective the generic medication is in managing the patient'south arrhythmia.
ten . Which of the following is a recommendation with regard to the use of generic medications for antiarrhythmic therapy?
| A) | Always switch to generic drugs for life-threatening arrhythmias and other arrhythmias that cause loss of consciousness. | ||
| B) | Limit apply of generic drugs to time-release preparations of mutual antiarrhythmic medications simply. | ||
| C) | When selecting a generic medication, give preference to one that has only ane preparation available and is readily available from pharmacies. | ||
| D) | Never use generic medications for less serious arrhythmias even if a reliable analysis is available and stable therapeutic drug levels tin can exist obtained. |
ESTABLISHING THE MEDICAL Programme OF Intendance
Practically speaking, the use of a generic commutation means that the patient could experience different effectiveness with different preparations. Random switching from proprietary to generic or from one generic to some other could increase side effects, decrease rate control, and crusade more than frequent relapse from normal sinus rhythm to atrial fibrillation. For that reason, any generic exchange of antiarrhythmic medications should be done very carefully. With many antiarrhythmic medications, very small variations in the serum blood level may influence the effectiveness of the medication in controlling the arrhythmia and significantly increase the gamble of proarrhythmias and serious side effects. Physician groups have made the following recommendations regarding the use of generic antiarrhythmic medications [39]:
-
Regardless of the preparation used, closely monitor the patient's status and serum drug levels. Adapt dosage as indicated by data.
-
Avoid substitution of antiarrhythmic medications for patients with life-threatening arrhythmias, arrhythmias that crusade loss of consciousness, or when a change in drug level (increase) tin can crusade life-threatening proarrhythmias.
-
Employ a generic for less serious arrhythmias if an easy, reliable assay is bachelor and a therapeutic drug level is stable and sustained over time.
-
If generic exchange is necessary, requite preference to generic medications that have but one preparation available, thus avoiding multiple switches from ane generic product to another. Likewise requite preference to a generic preparation that is widely bachelor in infirmary and outpatient pharmacies.
-
If switching from a proprietary to a generic medication, re-establish effectiveness and proper dose with the new grooming.
-
The physician may wish to specify on the prescription the exact grooming of a medication to be dispensed. Some states have regulations that limit the physician'due south ability to specify preparations. Also, specifying a proprietary medication may present a financial issue for the patient; insurance companies may not cover the higher cost preparation.
xi . For persons in atrial fibrillation with a rapid ventricular response, the usual Iv bolus dose of diltiazem is
| A) | 0.25 mg/kg. | ||
| B) | 375 mcg/kg. | ||
| C) | 1 mg/kg. | ||
| D) | iii mg/kg. |
PHARMACOLOGIC THERAPY FOR RATE Command
Diltiazem acts past blocking calcium transport into the myocardial and vascular smooth muscle cells. As a upshot, conduction through the SA and AV nodes is slowed, and the refractory period of the AV node is prolonged. Ventricular rate is slowed, but the underlying atrial arrhythmia is non corrected. Diltiazem should be administered initially every bit an IV bolus. The usual dose is a 0.25 mg/kg bolus administered over a two-minute catamenia. Diltiazem has a rapid onset of action. If effective, it should dull the patient'south heart rate within three to vii minutes of administration. If the initial dose is ineffective in slowing the patient's eye rate, the bolus may exist repeated at a higher dose of 0.35 mg/kg over two minutes. The patient's eye rate and rhythm and blood force per unit area should be monitored during administration. Bradycardia, bradyarrhythmias such as heart block, and hypotension may occur. To achieve or maintain rate control, a continuous infusion may be started following bolus administration. The infusion may exist started at 10 mg/hour and increased in increments of 5 mg/60 minutes to achieve rate control if no undesirable side effects occur. Diltiazem should be used with caution in patients with congestive center failure, known pre-existing conduction defects, and pregnant hypotension [3,26,54].
12 . Which of the post-obit statements is TRUE regarding the employ of esmolol in atrial fibrillation?
| A) | Esmolol has a very brusk half-life. | ||
| B) | Esmolol stimulates an increase in SA node firing. | ||
| C) | Esmolol comes in both intravenous and oral preparations. | ||
| D) | The initial loading dose of esmolol is 0.05 mg/kg over ane minute. |
PHARMACOLOGIC THERAPY FOR RATE CONTROL
Esmolol is a short-interim beta adrenergic blocker that slows ventricular rate in atrial fibrillation by slowing conduction through the AV node. Initial administration is an IV bolus dose/loading dose of 0.5 mg/kg administered over one infinitesimal. The bolus should be followed with an infusion of 0.05 mg/kg/min for four minutes. If the desired rate control is achieved, the infusion should exist connected at that rate. If adequate charge per unit command is not achieved at that dose, the bolus should be repeated followed by an infusion of 0.1 mg/min for four minutes. The total dose should not exceed 200 mcg/kg/min. This procedure may be repeated until rate control is achieved or undesirable side furnishings occur. The patient'south eye rate, ECG rhythm, and blood pressure should be monitored during the administration. Hypotension may occur. Once rate control is achieved, the infusion should be reduced to 0.025 mg/kg/min. Because esmolol has a short one-half-life, the therapeutic effects and side effects ordinarily reverse within ten to xx minutes after the infusion is stopped. Considering the therapeutic effects article of clothing off quickly, care should be taken when switching the patient to an oral preparation to prevent relapse/loss of charge per unit control. To transition the patient to oral medication, the first dose of the oral medication should be administered while the patient is still receiving esmolol. Thirty minutes afterwards the beginning oral dose is given, the esmolol infusion should be reduced by half. The second dose of the oral agent should exist administered at its scheduled time. I hr after the scheduled administration of the 2d oral dose, appraise the patient's heart rate, ECG rhythm, and blood pressure. If charge per unit control is maintained, the esmolol infusion may be discontinued. Note: Esmolol has no oral preparation; long-term command by oral agent requires a different agent [3,26].
xiii . A drug of choice for chronic rate control for persons in atrial fibrillation who likewise have an active lifestyle is
| A) | esmolol. | ||
| B) | warfarin. | ||
| C) | diltiazem. | ||
| D) | adenosine. |
PHARMACOLOGIC THERAPY FOR Rate CONTROL
Oral diltiazem preparations provide chronic rate command in atrial fibrillation and are a drug of choice for persons who have a physically agile lifestyle; even so, their use for rate control is unlabeled [26]. Diltiazem comes in immediate- and extended-release forms. Firsthand-release doses must be taken iii to four times per day; extended-release forms require only daily dosing. In improver to bradycardia, hypotension, and eye cake, other side effects of oral diltiazem include flushing, angina, insomnia, headache, nausea, syncope, and signs of congestive centre failure. Care should be taken when diltiazem is combined with negative inotropic drugs, other calcium channel blockers, and digoxin. Combination therapy increases the hazard of bradycardia, conduction abnormalities, hypotension, and signs of congestive centre failure [three,26,54].
14 . Severe digitalis toxicity may be reversed past the use of
| A) | flecainide. | ||
| B) | amiodarone. | ||
| C) | digoxin allowed Fab. | ||
| D) | oral magnesium salts. |
PHARMACOLOGIC THERAPY FOR RATE Control
Oral digoxin may be the first drug of choice for patients with atrial fibrillation and congestive middle failure caused by systolic dysfunction [62]. Digoxin does not provide acceptable charge per unit control during exercise or activity. The usual oral maintenance dose of digoxin is 0.125–0.25 mg daily. Serum digoxin levels should be monitored periodically. The patient should exist monitored for signs of digitalis toxicity. The patient and/or family unit should be taught to recognize fundamental signs of toxicity. These include nausea and airsickness, headache, unexplained weakness, malaise, and visual disturbances every bit well as slow centre rate. ECG changes that may occur with digitalis toxicity include sinus bradycardia, heart cake, and multiple tachyarrhythmias. Digitalis toxicity is more likely to occur in elderly persons. Electrolyte imbalances such as hypokalemia, renal failure, or combined therapy with other antiarrhythmic agents may potentiate the effects of digoxin and increase the take chances of digitalis toxicity. Severe digitalis toxicity may be treated with digoxin immune Fab [26].
15 . Of the post-obit, which argument most accurately describes the action of ibutilide?
| A) | Ibutilide slightly increases the sinus rate. | ||
| B) | Ibutilide accelerates the duration of the action potential. | ||
| C) | Ibutilide prolongs the elapsing of the ventricular refractory catamenia. | ||
| D) | Ibutilide blocks move of calcium ions through all calcium channels. |
PHARMACOLOGIC CARDIOVERSION
Ibutilide is a drug approved by the FDA for the acute termination of atrial fibrillation and atrial palpitate. A Course III antiarrhythmic, ibutilide acts by prolonging the duration of the action potential, the atrial refractory period, and the ventricular refractory period. It also slightly slows the sinus rate and conduction through the AV node. Ibutilide's exact mechanism of action is unknown [26]. On a cellular level, it has been reported to exert its effects by both blocking repolarizing potassium currents and initiating an inward depolarizing sodium current [49]. Ibutilide does not appear to have a direct impact on cardiac output, simply monitoring for conduction disturbances and heart block is recommended [26].
sixteen . The treatment of choice for torsades de pointes is
| A) | Iv atropine. | ||
| B) | IV lidocaine. | ||
| C) | 4 dopamine. | ||
| D) | IV magnesium sulfate. |
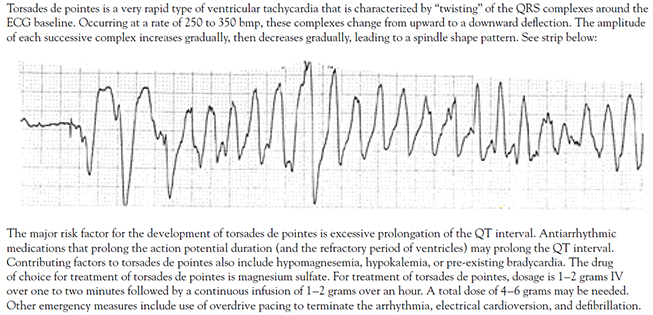
PHARMACOLOGIC CARDIOVERSION
TORSADES DE POINTES
| |
17 . Prior to initiating dofetilide therapy, other antiarrhythmic medications that prolong the QT interval should be
| A) | switched from oral preparations to IV. | ||
| B) | reduced by one-half for the offset two days of dofetilide therapy. | ||
| C) | discontinued three or more half-lives earlier dofetilide is begun. | ||
| D) | gradually increased in dose until maximum effectiveness is reduced. |
PHARMACOLOGIC CARDIOVERSION
For persons with normal renal function, studies have shown that an oral dose of 500 mcg twice daily is constructive in restoring normal sinus rhythm. In the presence of impaired renal office, the dose should be reduced. The dose may be as low every bit 125 mcg twice daily. The usual range for the maintenance dose (normal renal function) is 125–500 mcg twice daily. The administration protocol is as follows [26]:
-
Admit the patient to an inpatient facility approved for dofetilide administration.
-
Antiarrhythmic medications associated with prolonged QT interval (primarily Class I, Class III) should be discontinued; the guideline is at least three or more one-half-lives before initiation of dofetilide. Digoxin, beta blockers, and calcium channel blockers may exist used to control ventricular rate during the withdrawal flow of these drugs.
-
Electrolyte imbalances, especially potassium and magnesium, should exist corrected before therapy is initiated.
-
QTc should exist monitored periodically. Dosage should be reduced if QTc increases by more than fifteen% or exceeds 500 msec inside two to three hours after initial dose. Meet the package labeling or website information for specific dosage reduction guidelines.
-
Later on the second dose, if the QTc interval is greater than 500 msec (or 550 msec in the presence of ventricular conduction abnormalities), dofetilide should be discontinued.
-
Go on ECG monitoring for a minimum of three days.
18 . One toxic result associated with amiodarone administration is
| A) | pericarditis. | ||
| B) | endocarditis. | ||
| C) | pulmonary fibrosis. | ||
| D) | elevated hemoglobin. |
PHARMACOLOGIC CARDIOVERSION
Amiodarone has received increasing attention for its ability to catechumen atrial fibrillation to normal sinus rhythm and to preclude atrial fibrillation in patients undergoing CABG surgery [47,50,seventy]. Categorized equally a Form Three antiarrhythmic, amiodarone has properties of all four classes. It inhibits conduction through the AV node, prolongs the activity potential and refractory period, and inhibits adrenergic stimulation. It may exist safely used for patients with congestive middle failure, coronary artery disease, and persons with accessory pathway conduction. The proarrhythmic furnishings associated with amiodarone include bradycardia, heart block, ventricular fibrillation, and ventricular tachycardias including torsades de pointes. Hypotension may occur with IV assistants. Amiodarone is associated with multiple severe side effects and toxic furnishings. These include pulmonary fibrosis, impaired vision from corneal deposits, photosensitive pare, thyroid dysfunction, and liver dysfunction [26,71]. Prior to beginning amiodarone therapy, the physician should obtain baseline data. Appropriate data includes a chest x-ray, pulmonary function tests, thyroid part tests, liver function tests, and renal studies. Patients receiving amiodarone therapy should be monitored for development of toxic effects. Serious toxicity, including death due to bradycardia catastrophe in cardiac arrest, has been reported [3,26]. If symptoms develop, amiodarone should be discontinued. Utilize of the lowest effective maintenance dose is highly recommended due to the multitude and severity of side effects associated with amiodarone therapy. The FDA has issued a warning regarding concurrent use of amiodarone and simvastatin [72]. In patients who are taking both simvastatin and amiodarone, the dose of simvastatin should not exceed xx mg/twenty-four hour period [26,68].
xix . Electrical cardioversion is the handling of pick for persons with
| A) | loss of accessory pathway conduction. | ||
| B) | stable atrial fibrillation at a controlled charge per unit. | ||
| C) | hemodynamically unstable atrial fibrillation. | ||
| D) | presence of atrial thrombus documented by TEE. |
ELECTRICAL CARDIOVERSION
Electrical cardioversion has been establish to exist an constructive and safe method for restoring normal sinus rhythm in a number of patients. Information technology is the treatment of choice for persons with hemodynamically unstable atrial fibrillation [23]. Electrical cardioversion is also indicated for:
-
Persons for whom in that location is a reasonable expectation that normal sinus rhythm tin can exist restored and maintained
-
Persons who require "atrial kick" to salve incapacitating or unpleasant symptoms, improve exercise tolerance, and increase their power to perform their usual daily activities
-
Persons who would benefit from normal sinus rhythm but who have non been able to be successfully cardioverted pharmacologically (i.e., "failed" pharmacologic cardioversion)
20 . Prior to electrical cardioversion, a patient should be adequately anticoagulated with an INR maintained between 2.0-3.0 for
| A) | 48 hours. | ||
| B) | 3 weeks. | ||
| C) | ane month. | ||
| D) | 3 months. |
Electric CARDIOVERSION
As indicated, anticoagulation before cardioversion is required for most patients. Warfarin should be initiated, and the dose adapted to reach and maintain a target goal of an international normalized ratio (INR) of 2.5 (range: 2.0 to three.0). Later on the patient has been maintained for three weeks at that therapeutic goal, cardioversion may be performed. Following cardioversion, anticoagulation should exist maintained for four weeks. Equally an alternative, patients who need cardioversion may be admitted to the hospital and placed on a heparin drip [75]. A TEE is performed to rule out the presence of atrial thrombi. If no thrombi are present, the cardioversion may be safely performed [23]. Post-obit the procedure, the patient should receive anticoagulation therapy for 4 weeks [3,6,76,77].
21 . Because it prolongs the QT interval, disopyramide therapy is Non recommended equally a starting time drug of choice for patients with
| A) | asthma. | ||
| B) | anemia. | ||
| C) | hypertension. | ||
| D) | shortened QT interval. |
PHARMACOLOGIC THERAPY FOR MAINTENANCE OF NORMAL SINUS RHYTHM
Disopyramide may exist administered in a brusque- or sustained-release course. Because it prolongs the QT interval, it is not indicated as a start drug of option for persons who also accept hypertension. The combination of left ventricular hypertrophy caused by long-standing hypertension and a prolonged QT interval greatly increases the adventure of proarrhythmias such as torsades de pointes [26].
22 . The drug of choice for treatment of atrial fibrillation that has no identifiable cause is
| A) | quinidine. | ||
| B) | flecainide. | ||
| C) | amiodarone. | ||
| D) | propafenone. |
PHARMACOLOGIC THERAPY FOR MAINTENANCE OF NORMAL SINUS RHYTHM
Flecainide is the drug of pick for the handling of atrial fibrillation that has no identifiable cause. It is recommended for use only in the absence of structural middle disease and should not be prescribed for patients who have had a recent myocardial infarction or accept aberrant left ventricular function [26].
23 . For persons at low chance for cerebrovascular accident, the recommended anticoagulation therapy is
| A) | watchful waiting. | ||
| B) | warfarin 1-5 mg daily. | ||
| C) | aspirin 81-325 mg daily. | ||
| D) | clopidogrel 300 mg weekly. |
PREVENTION OF THROMBOEMBOLIC COMPLICATIONS
Two medications used commonly in prevention of thromboembolic events are aspirin and warfarin. They may be used singly or in combination to prevent thromboembolic events, simply the efficacy of combination therapy has not been established [117,118,119,120]. Warfarin in comparison to aspirin leads to a 39% relative risk reduction in stroke [121]. Rather than adding aspirin, the AHA/ACC/HRS Task Force recommends increasing the intensity of the anticoagulant to a maximum target INR of 2.0–3.0 [23]. Aspirin acts by decreasing platelet assemblage. Because information technology offers only pocket-sized protection confronting stroke for patients with atrial fibrillation, its use is recommended for low-take a chance patients [42]. Equally noted, low-risk patients include those who are younger than 65 years of age and have no underlying cardiac disease. It may as well be recommended for utilise in patients, particularly elderly persons, who cannot safely take warfarin. The usual adult dose of aspirin is 81–325 mg oral per twenty-four hours [42].
24 . Which of the following is an indication for radiofrequency ablation of the AV node?
| A) | Failed electrical or pharmacologic cardioversion | ||
| B) | Asymptomatic atrial fibrillation at a controlled rate | ||
| C) | Adequate charge per unit control with oral antiarrhythmic medications | ||
| D) | Absenteeism of side effects or proarrhythmic effects with oral antiarrhythmic therapy |
RADIOFREQUENCY ABLATION IN THE Management OF ATRIAL FIBRILLATION
Radiofrequency ablation of the AV node is an interventional therapy commonly used in the direction of atrial fibrillation. It has been found to be effective for persons who have significant symptoms with atrial fibrillation and/or poorly controlled ventricular rate who also [iii,19,42,52,134]:
-
Have remained in atrial fibrillation despite attempts at electrical or pharmacologic cardioversion or who quickly revert to atrial fibrillation following cardioversion (referred to as "failed cardioversion")
-
Cannot have antiarrhythmic medications considering of severe side furnishings or the development of proarrhythmias
-
Have inadequate rate control despite optimal dosing of advisable antiarrhythmic agents
25 . Which of the following interventions represent appropriate intendance following electrophysiology testing?
| A) | Administrate Four heparin past continuous infusion for 24 hours. | ||
| B) | Maintain the patient in Trendelenburg position until ablation sheaths are removed. | ||
| C) | Proceed the patient NPO for 24 hours or until bowel sounds render following the procedure. | ||
| D) | Appraise sites used for insertion of the electrophysiology catheters for bleeding or hematoma formation. |
RADIOFREQUENCY ABLATION IN THE Management OF ATRIAL FIBRILLATION
ELECTROPHYSIOLOGY TESTING AND ABLATION
| Electrophysiology testing is an invasive diagnostic cardiovascular procedure that tin can confirm and pinpoint the location of accessory conduction pathway(s) in the heart. It is performed in a peculiarly equipped cardiac catheterization lab. Multiple catheters are introduced through the femoral vein into the right side of the middle. Each catheter contains 4 to sixteen electrodes for monitoring the center's electrical activity. The catheters are positioned in various locations in the heart; common sites include the high correct atrium, the noon of the right ventricle, the AV junction/His bundle, and the coronary sinus. During the procedure, specific pacing protocols are used to determine the location and characteristics of whatsoever accessory pathways. Based on the location and characteristics of the accessory pathway(southward), a handling program volition be adult. Handling options include medical management of the syndrome and associated arrhythmias and radiofrequency ablation of the accessory pathway(s). Radiofrequency ablation is gaining in popularity as the treatment of selection. If the pathway is to exist ablated, another catheter (the ablation catheter) is advanced to the site, and more extensive/precise mapping of the accessory pathway is done. The location of the ablation catheter is adjusted until it is close to the pathway. Radiofrequency energy waves are applied to the pathway until pre-excitation disappears and the tachyarrhythmia cannot exist restarted. The procedure may last from three to five hours. Postprocedure care involves bedrest, frequent vital signs, and observing the femoral insertion sites for bleeding or hematoma formation. |
26 . The recommended postoperative therapy to foreclose atrial fibrillation in patients who have undergone coronary artery bypass graft surgery is administration of
| A) | beta blockers. | ||
| B) | synchronized cardioversion. | ||
| C) | continuous infusion of esmolol. | ||
| D) | intermittent 4 boluses of diltiazem. |
Direction OF ATRIAL FIBRILLATION FOLLOWING CORONARY ARTERY Featherbed GRAFT SURGERY
For prevention of postoperative atrial fibrillation following CABG, many sources recommend that beta-blocker therapy exist initiated (or resumed) every bit shortly as reasonably possible following surgery [147,149]. Safety employ of other antiarrhythmic agents has non been recommended. Use of digoxin has been found to have piffling effect and is non recommended [23]. Preoperative administration of statins may reduce postoperative atrial fibrillation and shorten the patient'south stay on the ICU and in the hospital [150,151]. Studies have found that the postoperative prophylactic use of amiodarone reduces the risk of atrial fibrillation and decreases the full price of care [149,152,153]. However, the employ of amiodarone and other antiarrhythmic medications, such as calcium channel blockers and procainamide has been linked to the unacceptable side effects of bradycardia and hypotension [lxx]. Early intervention for patients who develop increasingly frequent premature atrial contractions (PACs) has been recommended past some clinicians. Notwithstanding, there is no consensus regarding the best handling. Immediate intervention is indicated for persons who develop atrial fibrillation in the postoperative menses. Based on an assessment of the patient's symptoms and hemodynamic status, medical handling may involve intravenous medications for rate command, pharmacologic cardioversion, or electric cardioversion along with identification and correction of factors that may contribute to the development of arrhythmias such as hypokalemia and hypomagnesemia. If atrial fibrillation persists despite appropriate therapy, anticoagulation therapy should be started as presently as the surgeon decides information technology is feasible. Iv to 6 weeks following surgery, the patient's status should be re-evaluated [154,155]. In some persons, postoperative atrial fibrillation is a relatively transient arrhythmia that may spontaneously resolve. However, for others, the arrhythmia may persist and require medical management equally previously described for persons with coronary avenue illness [3].
27 . ECG characteristics associated with Wolff-Parkinson-White syndrome include
| A) | normal ST moving ridge. | ||
| B) | prolonged PR interval. | ||
| C) | regular rate and rhythm. | ||
| D) | presence of a delta wave. |
ATRIAL FIBRILLATION IN WOLFF-PARKINSON-WHITE SYNDROME
During early on fetal development, a number of bundles of fibers exist that connect the atria and ventricles. As fetal development progresses, these connections disappear until the AV node is left as the only functional electrical connection between atria and ventricles. In persons with WPW, i or more of these fibrous connecting bundles has persisted into machismo. The connecting package, chosen an accessory pathway, provides an alternative route for the conduction of an electrical impulse through the centre. Depending on a number of factors, an electric impulse may travel simply through the normal conducting pathway, through both the normal and the accessory pathways, or only through the accessory pathway. When the impulse travels through the accessory pathway, it bypasses the normal delay in the AV node and reaches the ventricles early. It initiates ventricular depolarization before the impulse traveling down the normal conduction pathway can attain the ventricles. The ventricles depolarize abnormally. The abnormal conduction through the accessory pathway alters the normal ECG waveform. The changes include [156]:
-
A shortened PR interval (less than 0.12 sec). The PR interval is shortened because the impulse from the atria reaches the ventricles through the accessory pathway more rapidly than normal.
-
The presence of a delta wave. A delta moving ridge is a slurring of the initial deflection (either positive or negative) of the QRS complex. It reflects the early, aberrant depolarization of the ventricles that occurs when the impulse travels through the accessory pathway.
-
An abnormally widened QRS circuitous (greater than 0.12 sec). The widened, abnormal QRS occurs when nigh of the ventricular depolarization is stimulated past an impulse traveling downward the accompaniment pathway. In normal conduction, both ventricles depolarize almost simultaneously. With accompaniment pathway conduction, 1 ventricle is stimulated to depolarize earlier the other.
-
Aberrant ST waves. An ST wave represents repolarization. When depolarization is abnormal, the design of repolarization volition as well be abnormal.
28 . The initial drug of selection for the management of atrial fibrillation in Wolff-Parkinson-White syndrome is
| A) | digoxin. | ||
| B) | warfarin. | ||
| C) | verapamil. | ||
| D) | procainamide. |
ATRIAL FIBRILLATION IN WOLFF-PARKINSON-WHITE SYNDROME
When a patient with WPW presents with atrial fibrillation with rapid ventricular response, the patient'south hemodynamic condition should be immediately assessed. If there are signs of hemodynamic instability, such as hypotension, signs of congestive middle failure, or ischemic chest pain, the patient should be immediately cardioverted [156]. If the patient is non hemodynamically unstable, charge per unit control is the loftier priority. Appropriate medication selection is critical. Use of medications such every bit verapamil and digoxin that deadening or block conduction through the AV node will beal atrial fibrillation in WPW and run the risk of accelerating the ventricular rate to the point that ventricular fibrillation can occur [26,156]. The treatment of pick for hemodynamically stable patients with atrial fibrillation and WPW is IV procainamide or ibutilide [23]. Verapamil, diltiazem, adenosine, digoxin (oral or intravenous), and intravenous amiodarone can precipitate ventricular fibrillation and should not be used [23].
29 . In the case report of astute-onset atrial fibrillation, Patient D's risk factors for the development of atrial fibrillation include
| A) | palpitations. | ||
| B) | hypertension. | ||
| C) | alcoholic cirrhosis. | ||
| D) | gastroesophageal reflux. |
SIMULATED Instance Study: THE PATIENT WITH Astute-ONSET ATRIAL FIBRILLATION
Patient D is a human, 68 years of age, who presents to the emergency department late 1 evening complaining of increasing shortness of breath, dizziness, and the sensation of his "middle racing." On admission, his heart rate is 160 bpm, claret pressure level 100/l mm Hg, respirations 26 breaths per infinitesimal, and oxygen saturation 88% on room air. Patient D says that his symptoms started abruptly earlier that twenty-four hours and have steadily go worse. He reports a history of long-continuing hypertension, coronary avenue disease, and a recent percutaneous transluminal angioplasty with placement of 2 stents.
Comments and rationale : Symptoms such equally those Patient D presents are common indications of acute onset atrial fibrillation with rapid ventricular response. His past medical history is positive for risk factors for the development of atrial fibrillation. These include a positive cardiac history, with hypertension and coronary artery disease, as well as increasing age.
xxx . A QTc interval of 580 msec is
| A) | within normal limits. | ||
| B) | dangerously shortened. | ||
| C) | significantly prolonged. | ||
| D) | acceptable for patients on antiarrhythmic therapy. |
Faux Instance Written report: CLINICAL Direction OF THE PATIENT WITH PERSISTENT ATRIAL FIBRILLATION
Laboratory tests are ordered, including a complete blood count, serum electrolytes, renal and hepatic function tests, breast x-ray, urinalysis, and PT/INR. A peripheral IV admission is established. Continuous telemetry monitoring is maintained, and Patient Due west'south vital signs are monitored every four hours. Her heart rate remains between 48 and 52 bpm. Patient Westward's oral antiarrhythmic medication is discontinued. Assay of Patient Due west's ECG shows that her QTc is 580 msec. The therapeutic options of electrical cardioversion, radiofrequency ablation, and continued oral antiarrhythmic therapy with insertion of a demand pacemaker are discussed with the patient. The patient expresses reluctance to undergo pacemaker insertion at this fourth dimension. With the patient's agreement, she is scheduled for electrical cardioversion. Patient W expresses the agreement that pacemaker insertion or ablation with pacemaker insertion might however be required if cardioversion is unsuccessful or if she becomes symptomatic on therapy to maintain sinus rhythm following successful cardioversion.
Comments and rationale : Laboratory tests such as a consummate blood count and serum electrolytes are ordered to rule out any abnormal findings (e.g., anemia, hypokalemia, hypomagnesemia) that may make management of atrial fibrillation more difficult. Due to Patient W's continued bradycardia, her oral antiarrhythmic medication is discontinued. Her QTc interval is significantly prolonged. Considering Patient W has adult bradycardia and a prolonged QTc interval, she is not a candidate for pharmacologic cardioversion.
Source: https://www.netce.com/studypoints.php?courseid=2093;printable=yes;page=printquestions
0 Response to "what atrial wall muscle fails to contract in a synchronous fashion during atrial fibrillation?"
Post a Comment